Sponsorship review:
CTIMPs and interventional studies should be submitted for sponsorship review to the JRES regulatory team. Once all documents are reviewed the sponsorship process will take 2-4 weeks.
The following documents will be required to facilitate sponsorship:
- Protocol
- Participant Information Sheet
- Informed consent form
- Other patient-facing documents
- Funding agreement
- Evidence of public database registration (if applicable)
- Evidence of EudraCT registration (if applicable)
- Investigator brochure/SmPCs (if applicable)
- Delegation of Duties Signed Agreement
- Study Registration Questionnaire
- Data Protection Impact Assessment form
- Insurance Policy
SOP - Sponsorship and Governance Processes for International Studies at City St George’s, University of London
JRESGOVSOP0003 (CTIMPs)
JRESGOVSOP0028 (non-CTIMPs)
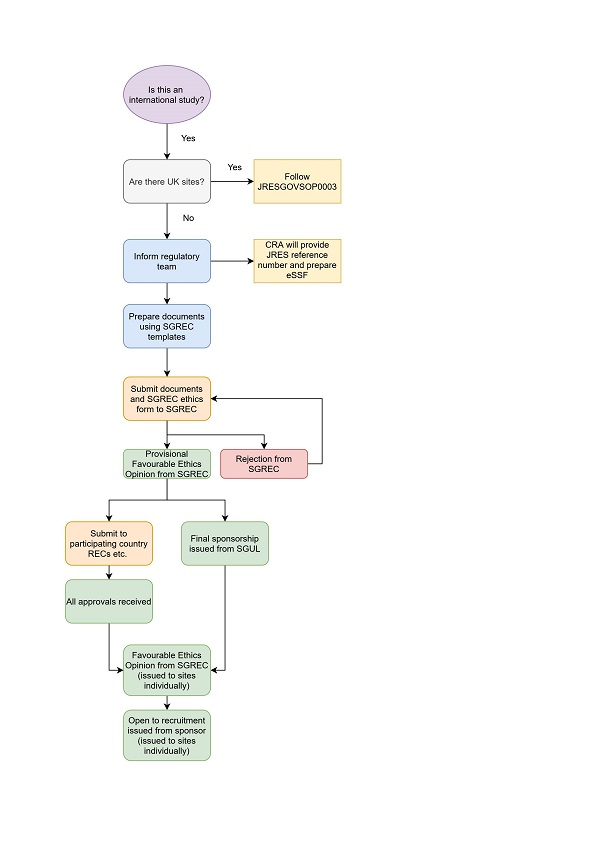
Research ethics review
The application will need to be submitted to St George’s Research Ethics Committee (SGREC) first and then to the participating country/countries local Research Ethics Committee(s). The Ethics Committee will consider the general ethical aspects of your project and it will review the safety aspects, the insurance cover and reputational risk to the University.
International studies collecting primary individual data subject to confidentiality considerations and without a named collaborator in each of the participating countries will not be considered by SGREC unless a local ethics approval has already been obtained from those countries for the same protocol.
The international study as a high-risk study should be led by the Supervisor (Academic staff), study team should have qualitative study expert in the group (if relevant to the research).
SOP can be found on the policies section of the St George’s Research Ethics Committee (SGREC): Study Application and Review Processes
Research projects must comply with the UK’s legal requirements and the University`s ethical requirements, as well as the laws and regulations of the country where the study is taking place or where the data will be collected.
After SGREC provisional approval, you must seek local ethics favourable opinion and sponsor governance approval from the local organisation(s) where the research is taking place and any other local/national approvals required by that organisation and country. Evidence of these approvals must be provided to the SGREC.
Researchers are required to submit the following to the SGREC when they have received favourable ethics opinion for their project:
1. An End of Study notification when the study ends
2. An amendment application if changes to the study are needed, e.g.: amended participant documents, changes to the study design, study dates or study team.
More information on this can be found on the webpage.
Amendments should be submitted first to the local REC and after their approval, to the SGREC. It will be reviewed by the Research Ethics and Integrity Officer and the Chair or Deputy Chair of the SGREC. The changes must not be implemented by the study team until approval for the amendment has been received by SGREC and local REC.
For further with the documents please email the Research Ethics and Integrity Officer.
If you are travelling abroad you should ensure travel insurance is in place.
If your research involves the export of human tissue samples from overseas to CSGUL, you need to follow the Human Tissue Authority (HTA) regulation of the import/export of human tissue for research purposes. Even for countries that do not regulate human tissue research, as a minimum you will be expected to seek assurances from the host institute that the material is ethically sourced and check the consent given, especially if you will use identifiable samples.
For further help understanding SGUL HTA regulation please email the Research Ethics and Integrity Officer.